A dispersant for coating is a substance or chemical additive that is used to enhance the dispersion or suspension of solid particles in a liquid coating. Dispersants can improve the quality and performance of coatings by ensuring that the particles are evenly distributed, preventing agglomeration and settling. They can also improve the stability and durability of coatings, as well as their adhesion to surfaces.
1.The process of dispersion and re-aggregation of pigments
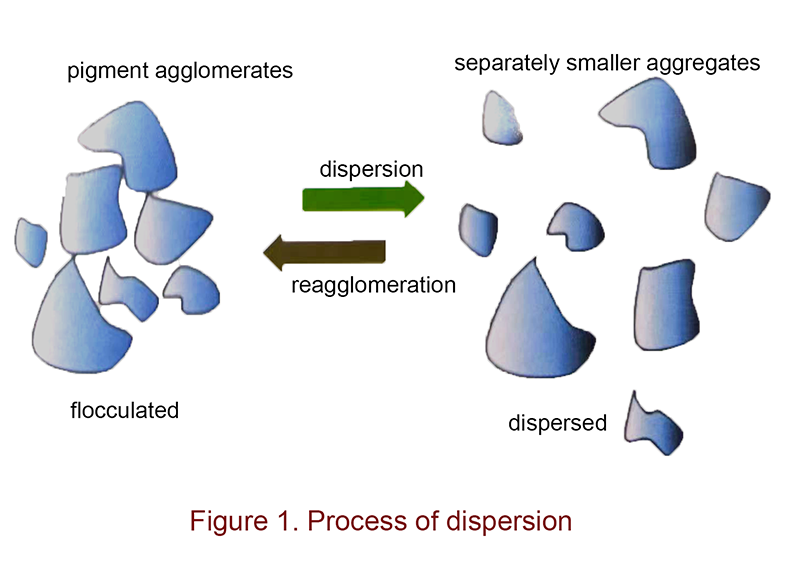
The dispersing process of pigments can generally be understood as a process in which pigments go from an aggregated state to smaller particles that are separated from each other. It is a process of decreasing particle size and increasing surface area. Usually, the total energy of the system during such a process increases due to the increase in surface energy. Therefore, external energy is needed to initiate the process, which is known as the grinding and dispersing process. In order to improve the efficiency of pigment dispersion, dispersing agents are commonly used. Without the help of dispersing agents, not only would more energy be required in the grinding process, but the dispersed pigment particles, with smaller particle sizes and higher surface energies, would have a tendency to spontaneously agglomerate and reform larger particles.
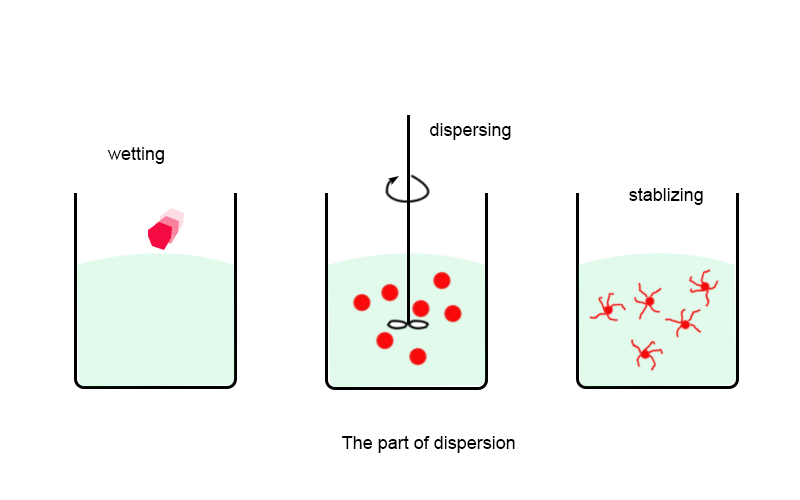
2.The three steps of pigment dispersion are:
3.Stabilization Mechanisms
According to the principles of physical chemistry, the stabilization of particles in a dispersed system is generally based on mutual repulsion between particles. Depending on the properties of the dispersant medium, there are generally two types of repulsion stabilization mechanisms: double layer stabilization and entropy stabilization.
3.1 Double-layer stabilization mechanism:
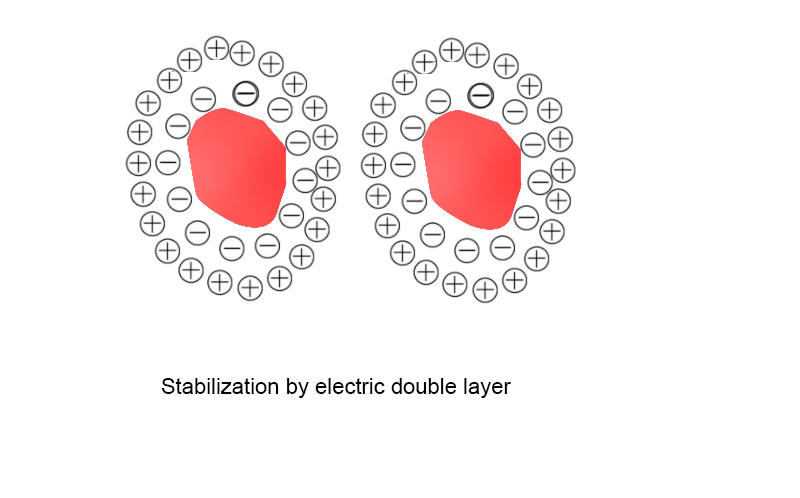
The double-layer stabilization mechanism mainly exists in waterborne systems, as shown in the figure left. Due to the high dielectric constant of water, when the surface of the pigment particles adsorbs the dispersant, the polar functional groups in the dispersant, which can be ionized, will be hydrolyzed to some extent on the pigment surface, thereby giving the pigment surface a certain charge.This charge is easy to attract some opposite charges around, thereby forming a double layer around the dispersed particles.
This double layer has a certain thickness with elasticity. When the particles come close to each other through Brownian motion, particles with the same charge of double layers will produce electrostatic repulsion between them. The more overlapping of the two double layers, the greater the repulsion force, but the charge of the double-layer structure is easily influenced by impurities and may collapse.
In waterborne systems, the dispersing medium is easy to ionize, and the double-layer structure is more easily effective, while in solvent-type systems, ionization phenomena are difficult to occur, and the double-layer is also difficult to form, thus its effect is relatively limited.
3.2 Entropy repulsion stabilization mechanism:
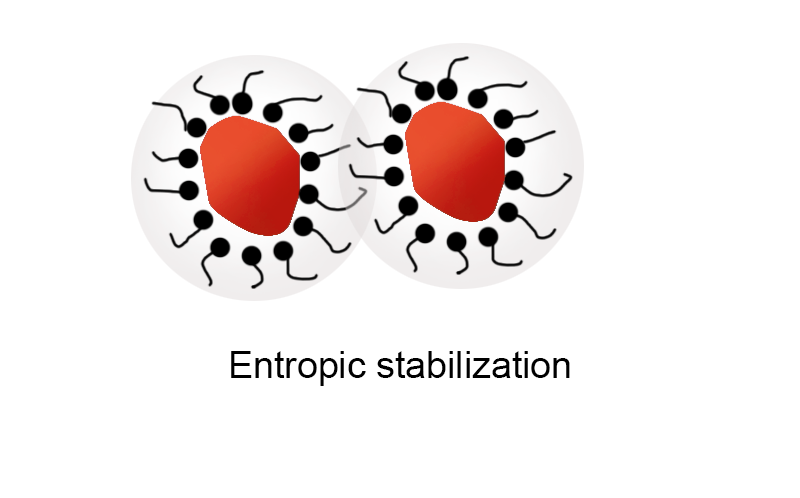
Entropy repulsion stabilization emerges as a critical mechanism in the field of polymer dispersants, functioning efficiently across both water-borne and solvent-based systems.
Its significance lies in its resilience; the stability of dispersion systems remains largely unaffected by fluctuations in pH levels and ionic strength. When applying a dispersant with a high molecular weight solvated chain to a pigment particle, it spreads out within the dispersion medium, forming a protective solvated polymer chain barrier. This barrier is paramount as it maintains particle separation, ensuring consistent dispersion quality and preventing particle aggregation, which is especially pronounced in the dispersion of organic pigments due to the steric hindrance provided by the mechanism.
In current paint formulation systems, water-based systems mostly adopt the double electric layer stabilization mechanism, while solvent-based systems mostly adopt the entropy-repulsion stabilization mechanism. With the increasing refinement of paint formulations, water-based systems are also increasingly using the entropy-repulsion mechanism to achieve storage stability under various complex conditions.
4. Classification of Dispersants
Single Anchoring or Multiple Anchoring
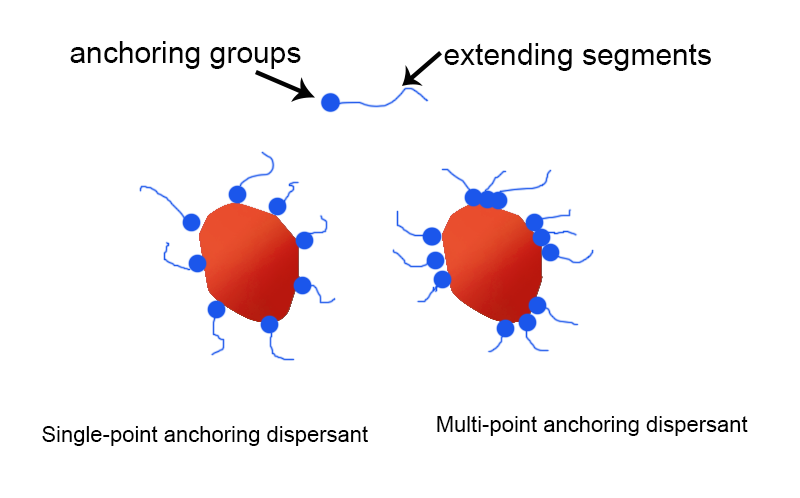
From the perspective of chemical structure, dispersants can usually be regarded as molecules similar to surfactants, consisting of anchoring groups and extending chain segments, as shown in the figure.
If a dispersant molecule has only one anchoring group, it is called a single-point anchoring dispersant. Most small-molecule wetting agents and some polymer-type dispersants belong to single-point anchoring dispersants. Generally speaking, single-point anchoring dispersants have more advantages in reducing the viscosity of the system.
On the other hand, if a dispersant molecule contains multiple anchoring groups, it becomes a multi-point anchoring dispersant. Some small-molecule wetting agents and most polymer-type dispersants belong to multi-point anchoring dispersants. Usually, due to the multiple adsorption points of multi-point anchoring dispersants, they have better storage stability.
Polymer-type dispersants
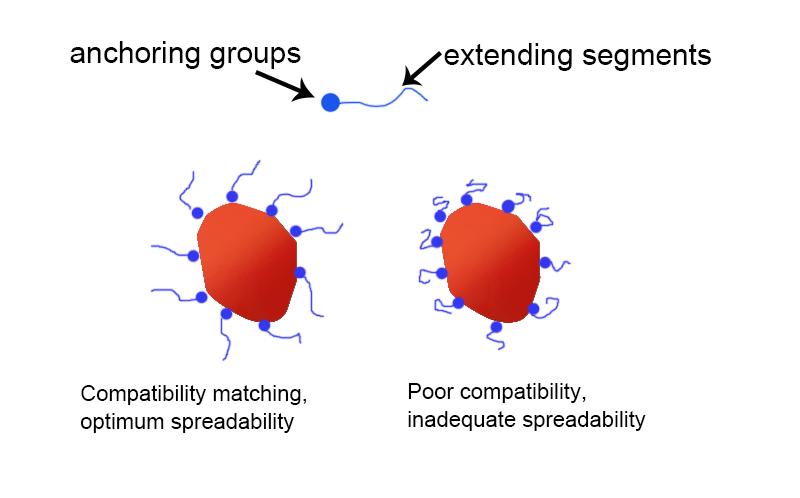
Polymer-type dispersants usually refer to dispersants with a molecular weight of more than 2000. From the perspective of chemical structure, the structure of polymer-type dispersants mainly depends on three aspects:
Among them, the type of anchoring group determines the type of pigment suitable for dispersion by the dispersant; the polarity of the solvation chain segment determines the resin system suitable for the dispersant, because for the dispersant to achieve the best effect, its solvation chain segment must have similar polarity to the system used, as shown in the figure below. In this case, the solvation chain segment of the dispersant has the best extension, which can maximize the prevention of mutual flocculation between pigment particles
There are various classification methods for polymer dispersants, but the most common are:
Sometimes, in order to increase the universality and efficiency of dispersants, multiple adsorption groups are introduced into the dispersant structure to improve its performance. For example, acid and basic groups can be simultaneously introduced into the dispersant structure, which can disperse both organic and inorganic pigments, achieving a good dispersion efficiency and avoiding floating color and blooming in some organic-inorganic pigment grinding systems.
For each polarity range of coatings, the corresponding dispersant with matching polarity must be used to achieve the best performance.
7. What we suggest for you based on different conditions
7.1 Low-polarity system
Low polarity systems typically contain low polarity solvents, resins, and other additives.
These systems often use low polarity solvents (like alkanes or aromatic solvents) and resins. Low polarity resins may include certain types of polyurethanes, epoxy resins, etc.
| Pigments Type | First Choice | Second Choice | Synergist |
| Titanium Dioxide | DCA-5723 | ||
| Matting powder | DCA-8417 | DCA-5722 | |
| Inorganic Pigments and Fillers | DCA-5723 | ||
| Red/Yellow Organic Pigments | DCA-8408 | DCA-8422 | |
| Phthalocyanine Blue/Green | DCA-8408 | DCA-8408 | DCA-8450 |
| Carbon Black | DCA-5722 | DCA-8408 | DCA-8450 |
| Mixed Pigments | DCA-805S |
7.2 Middle-polarity system
Midium polarity systems typically contain midium polarity solvents, resins, and other additives.
Resins:
-
Acrylic Resins: Widely used for water-based paints, known for versatility.
-
Vinyl Acetate-Ethylene (VAE) Copolymers: Commonly found in interior paints.
-
Styrene-Acrylic Resins: Offering good adhesion and water resistance.
Solvents:
-
Water: Primary solvent in water-based paints, environmentally friendly.
-
Glycol Ethers: Used as coalescents and viscosity reducers in water-based paints.
-
Propylene Glycol: Often added to control drying time in water-based coatings.
| Pigments Type | First Choice | Second Choice | Synergist |
| Titanium Dioxide | DCA-5122 | DCA-509 | |
| Matting powder | DCA-8432 | DCA-509 | |
| Inorganic Pigments and Fillers | DCA-509 | DCA-5155 | |
| Red/Yellow Organic Pigments | DCA-8330 | DCA-5172 | DCA-8422 |
| Phthalocyanine Blue/Green | DCA-8330 | DCA-8432 | DCA-8450 |
| Carbon Black | DCA-5421 | DCA-6431 | DCA-8450 |
| Mixed Pigments | DCA-805S |
7.3 Middle and high polarity system
Midium and high polarity systems typically contain midium and high polarity solvents, resins, and other additives.
Resins:
-
Polyester Resins: Used in industrial coatings for their durability.
-
Phenolic Resins: Known for their heat resistance and chemical stability.
-
Polyamide Resins: Used in high-performance coatings due to their toughness.
Solvents:
-
Ketones (e.g., MEK): Used as solvents in high-polarity coatings.
-
Toluene: Provides good solvency in paints and coatings.
-
| Pigments Type | First Choice | Second Choice | Synergist |
| Titanium Dioxide | DCA-509 | DCA-9520 | |
| Matting powder | DCA-6431 | DCA-9520 | |
| Inorganic Pigments and Fillers | DCA-509 | DCA-9520 | |
| Red/Yellow Organic Pigments | DCA-8330 | DCA-5172 | DCA-8422 |
| Phthalocyanine Blue/Green | DCA-8330 | DCA-9520 | DCA-8450 |
| Carbon Black | DCA-6431 | DCA-8330 | DCA-8450 |
| Anti-Flooding and Floating | DCA-805S |
7.4 Water based system
Resins:
Water-based paints use various types of resins, which provide different performance characteristics to meet various application needs. Here are some common types of resins used in water-based paints:
- Acrylic Resins: These are one of the most common types of resins used in water-based paints. Acrylic resins offer excellent weather resistance, adhesion, and water resistance. They are suitable for both interior and exterior coatings as well as decorative paints.
- Vinyl Acetate-Ethylene (VAE) Copolymers: VAE resins are typically used in interior water-based paints. They provide good abrasion resistance and adhesion, making them a common choice for interior wall paints.
- Styrene-Acrylic Resins: This resin type combines the properties of styrene and acrylic acid, offering good adhesion and chemical stability. They are commonly used in both decorative and functional water-based paints.
- Polyurethane Resins: Polyurethane resins are used in water-based paints to provide excellent wear resistance and chemical resistance. They are often used in wood coatings and floor paints.
- Acrylic Emulsions: These are water-based resin emulsions suitable for various coating applications. They provide outstanding weather resistance and water resistance.
- Polymer Emulsions: Polymer emulsions can include acrylic emulsions, vinyl emulsions, and others, used to manufacture various types of water-based paints, from wall paints to industrial coatings.
- Silicone Resins: Silicone resins are used in water-based paints to provide high-temperature resistance and chemical
| Pigments Type | First Choice | Second Choice | Synergist |
| Titanium Dioxide | DCA-666 | DCA-665 | |
| Matting powder | DCA-6431 | DCA-6411 | |
| Inorganic Pigments and Fillers | DCA-666 | DCA-665 | |
| Red/Yellow Organic Pigments | DCA-9496 | DCA-9491 | DCA-8422 |
| Phthalocyanine Blue/Green | DCA-9491 | DCA-9496 | DCA-8450 |
| Carbon Black | DCA-9491 | DCA-9496 | DCA-8450 |
| Anti-Flooding and Floating | DCA-805S |
7.5 Nosolvent system( UV-curing)
| Pigments Type | First Choice | Second Choice | Synergist |
| Titanium Dioxide | DCA-8115 | DCA-509 | |
| Matting powder | DCA-8430 | DCA-6431 | |
| Inorganic Pigments and Fillers | DCA-509 | DCA-9520 | |
| Red/Yellow Organic Pigments | DCA-8430 | DCA-6431 | DCA-8422 |
| Phthalocyanine Blue/Green | DCA-8430 | DCA-6431 | DCA-8450 |
| Carbon Black | DCA-8430 | DCA-6431 | DCA-8450 |